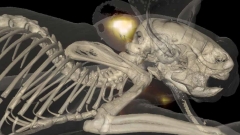
Figure: Nature Methods 9(6), 615–620 (2012). doi:10.1038/nmeth.2014.
The Laboratory develops advanced fluorescence imaging methods for pre-clinical and clinical imaging, leading in 2011 to the first clinical translation of a targeted fluorescent agent for optical molecular imaging of patients. Focus is on the development of quantitative, real-time and tomographic methods that go well beyond conventional “photographic” fluorescence image. To achieve this, we employ multi-spectral techniques and theoretical methods that solve for photon propagation into tissues and offer high fidelity imaging. In parallel we actively work on validating fluorescent agents and clinical targets. Particular focus areas are:
1. Real-time interventional fluorescence molecular imaging
Real-time interventional fluorescence molecular imaging in-particular for surgery and surgical endoscopy in order to improve lesion identification and accurate demarcation of cancer margins. Research is geared toward novel multi-spectral instrumentation and the use of appropriate fluorescent agents with clinical relevance (see Nat. Med. 2011 17:1315-9 and highlights below).
2. Hybrid fluorescence molecular tomography (FMT) and X-ray CT imaging:
The goal of the FMT group is to realize the full potential of the FMT-XCT hybrid imaging system and novel inversion methods using image priors toward accurate quantitative in-vivo molecular imaging in small animals. We further employ FMT-XCT in various preclinical applications to enable iresearch in basic research and drug discovery.
3. Fluorescence and color cryoslicing
Fluorescence and color cryoslicing for accurate measurement of the bio-distribution of fluorescent agents, labeled cells and tissue state. We similarly develop multi-spectral imaging approaches for automatic imaging of tissues during cryoslicing. Current research pursues hardware optimization and the development of imaging methods to correct for photon scattering and deliver images of superior quantification compared to simple photographic fluorescence approaches.
4. Standardization and benchmarking of fluorescence imaging systems.
We investigate the development of algorithms and phantoms for the standardization of fluorescence cameras. This involves validation of performance metrics, illumination correction, and design of benchmarking tests that would eventually allow for high-fidelity fluorescence molecular imaging.
5. Intravascular NIRF imaging
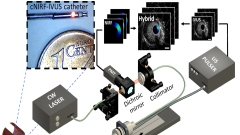
Figure: CBI
We integrate the development of a cNIRF-IVUS (c=corrected) catheter that enables simultaneous co-registered through-blood imaging of disease related morphological and biological alterations in coronary and peripheral arteries in vivo. We have developed 9F/15MHz peripheral and 4.5F/40MHz coronary hybrid catheters that we tested in swine and rabbit models. A correction algorithm utilizing IVUS information was developed to account for the distance-related fluorescence attenuation due to through-blood imaging.
Relevant publications
Bozhko D, Osborn EA, Rosenthal A, Verjans JWH, Hara T, McCarthy JR, Kellnberger S, Wissmeyer G, Ovespian SV, Mauskapf A, Stein AF, Jaffer FA, Ntziachristos V. Quantitative Intravascular Biological Fluorescence-Ultrasound Imaging of Coronary and Peripheral Arteries In Vivo. EHJCI, in press.
Teresa C., Maximilian K., Angelique A., Vasilis N., and Simon A. Patch-based anisotropic diffusion scheme for fluorescence diffuse optical tomography-part 2: Image reconstruction, Phys Med Biol 61(4), 1452-1475 (2016).
Koch M., and Ntziachristos V. Advancing surgical vision with fluorescence imaging, Annu Rev Med 67(1), 153-164 (2016).
Tjalma J. J., Garcia-Allende P. B., Hartmans E., Terwisscha van Scheltinga A. G., Boersma-van Ek W., Glatz J., Koch M., van Herwaarden Y. J., Bisseling T. M., Nagtegaal I. D., Timmer-Bosscha H., Koornstra J. J., Karrenbeld A., Kleibeuker J. H., van Dam G. M., Ntziachristos V., and Nagengast W. B. Molecular fluorescence endoscopy targeting vascular endothelial growth factor a for improved colorectal polyp detection, J Nucl Med 57(3), 480-485 (2016).
Mohajerani P., Koch M., Thürmel K., Haller B., Rummeny E. J., Ntziachristos V., and Meier R. Fluorescence-aided tomographic imaging of synovitis in the human finger, Radiology 272(3), 865-874 (2014).
Koch M., Glatz J., Ermolayev V., de Vries E. G. E., van Dam G. M., Englmeier K.-H., and Ntziachristos V. Video-rate optical flow corrected intraoperative functional fluorescence imaging, J Biomed Opt 19(4), 046012-046012 (2014).
Garcia-Allende P. B., Glatz J., Koch M., Tjalma J. J., Hartmans E., Terwisscha van Scheltinga A. G. T., Symvoulidis P., van Dam G. M., Nagengast W. B., and Ntziachristos V. Towards clinically translatable NIR fluorescence molecular guidance for colonoscopy, Biomed Opt Express 5(1), 78-92 (2014).
Glatz J., Garcia-Allende P. B., Becker V., Koch M., Meining A., and Ntziachristos V. Near-infrared fluorescence cholangiopancreatoscopy: Initial clinical feasibility results, Gastrointest Endosc 79(4), 664-668 (2014).
Glatz J., Varga J., Garcia-Allende P. B., Koch M., Greten F. R., and Ntziachristos V. Concurrent video-rate color and near-infrared fluorescence laparoscopy, J Biomed Opt 18(10), 101302-101302 (2013).
Correia T., Rudge T., Koch M., Ntziachristos V., and Arridge S. Wavelet-based data and solution compression for efficient image reconstruction in fluorescence diffuse optical tomography, J Biomed Opt 18(8), 086008-086008 (2013).
Garcia-Allende P. B., Glatz J., Koch M., and Ntziachristos V. Enriching the interventional vision of cancer with fluorescence and optoacoustic imaging, J Nucl Med 54(5), 664-667 (2013).
Ale A., Ermolayev V., Herzog E., Cohrs C., de Angelis M. H., and Ntziachristos V. FMT-XCT: In vivo animal studies with hybrid fluorescence molecular tomography-x-ray computed tomography, Nat Methods 9(6), 615-620 (2012).
Mallas G, Brooks DH, Rosenthal A, Nudelman RN, Mauskapf A, Jaffer FA, Ntziachristos V. Improving quantification of intravascular fluorescence imaging using structural information. Phys Med Biol 57(20), 6395-6406 (2012).
Scheuer W., van Dam G. M., Dobosz M., Schwaiger M., and Ntziachristos V. Drug-based optical agents: Infiltrating clinics at lower risk, Sci Transl Med 4(134), 134ps11-134ps11 (2012).
van Dam G. M., Themelis G., Crane L. M. A., Harlaar N. J., Pleijhuis R. G., Kelder W., Sarantopoulos A., de Jong J. S., Arts H. J. G., van der Zee A. G. J., Bart J., Low P. S., and Ntziachristos V. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-[alpha] targeting: First in-human results, Nat Med 17(10), 1315-1319 (2011).
Jaffer FA, Calfon MA, Rosenthal A, Mallas G, Razansky RN, Mauskapf A, Weissleder R, Libby P, Ntziachristos V. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol 57(25), 2516-2526 (2011).
Crane L. M. A., Themelis G., Pleijhuis R. G., Harlaar N. J., Sarantopoulos A., Arts H. J. G., van der Zee A. G. J., Vasilis N., and van Dam G. M. Intraoperative multispectral fluorescence imaging for the detection of the sentinel lymph node in cervical cancer: A novel concept, Mol Imaging Biol 13(5), 1043-1049 (2011).
Clinical translation of Fluorescence Molecular Imaging
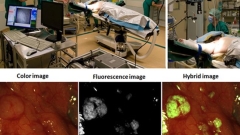
Figure: Phil. Trans. R. Soc. A 369(1955), 4666-4678 (2011). doi:10.1098/rsta.2011.0270. (top right) and Nature Medicine 17(10): 1315-1319 (2011). doi:10.1038/nm.2472. (bottom)
Our group translates novel molecular imaging methods for clinical interventional imaging, which is expected to shift the paradigm of disease detection within surgical and endoscopic procedures. The work seen in Fig.3 shows our fluorescence camera installed in the operating room (collaboration with Prof. Van Dam UMCG) and results from an ovarian cancer patient. The results show the integration of novel imaging modalities with the clinical translation of a fluorescent agent with targeting specificity to the folate receptor alpha; upregulated in ovarian cancer. This study (Nat. Med. 2011 17:1315-9) showed the first human use of targeted fluorescent dye in humans. Imaging is accomplished through a camera system that can detect and resolve color and NIR fluorescence in real-time. Translation of more agents is expected over the next years to address a larger number of diseases. In Science Translational Medicine we also described the challenges associated with the clinical translation of fluorescent agents and provided an overview of alternatives to confront these challenges and reduce the risk and investment required for interventional use. For further details see Scheuer et. al. Sci. Transl. Med. 2012 4:134ps11.
Multi-spectral fluorescence imaging and endoscopy
In many interventional procedures, such as surgery and diagnostic or surgical endoscopy, clinical decision making still relies on the visual appearance or palpation of various lesions and tissue areas.
Surgery is then confronted with difficulties in cancer spread identification and in the accurate delineation of the tumor margins, leading to incomplete resections of tumors that could compromise the patient prognosis.
Similarly, surveillance and endoscopic resection face low contrast between small cancerous lesions and benign lesions or surrounding tissue, and the lack of information on lesion depth that complicates detection or decision-making about the appropriate excision procedure. Multi-spectral fluorescence imaging and endoscopy evolve as a promising approach for interventional guidance. Increased detection sensitivity and specificity can be afforded by utilizing wide-field fluorescence imaging combined with targeted agents to enable the recognition of otherwise invisible disease biomarkers. For this reason current researches focuses on applying simultaneous color and NIR fluorescence detection in flexible endoscopes and laparoscopes that are routinely employed in clinical surveillance endoscopy and therapy monitoring procedures.
A multi-channel camera system was developed for intra-operative and endoscopic fluorescence imaging. The concurrent acquisition of color and NIR fluorescence images is facilitated by a dichroic mirror and a custom designed software. Video-rate processing of the data is enabled by GPU empowered computing. The system can easily be integrated into the operating room and surgical and surveillance procedures and has been employed for a number of clinical trials already.
Image management and analysis incorporates theoretical photon-tissue interaction models to provide accurate true quantitative fluorescence imaging, i.e. imaging that is independent of tissue optical properties, as well as normalization for system illumination and detection specifics. This leads to a minimization of false alarms and negatives. The combination of these acquisition and analysis approaches offer a new generation of fluorescence imaging for the clinics, to improve surgical and endoscopic vision.
FMT-XCT
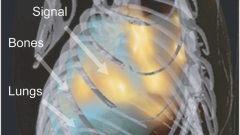
Figure: Nature Methods 9(6), 615–620 (2012). doi:10.1038/nmeth.2014.
Fluorescence imaging developments have also led to the development of hybrid fluorescence molecular tomography (FMT) X-ray CT (XCT) imaging (see Ale et. al., Nature Methods 2012 9(6), 615-620, 2012). These approaches are shifting the ways of detecting cellular and sub-cellular processes in animals and humans by virtually offering in vivo staining of important biomolecules that play a crucial role in disease progression or treatment. They can therefore lead to new exploratory, diagnostic and theranostic methods.