Godinho Lab - Publications
Notch-mediated re-specification of neuronal identity during central nervous system development
08 November 2021: Peter Engerer, Eleni Petridou, Philip R. Williams, Sachihiro C. Suzuki, Takeshi Yoshimatsu, Ruben Portugues, Thomas Misgeld, Leanne Godinho
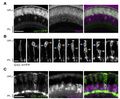
Neuronal identity has long been thought of as immutable, so that once a cell acquires a specific fate, it is main-tained for life.1 Studies using the overexpression of potent transcription factors to experimentally reprogram neuronal fate in the mouse neocortex and retina have challenged this notion by revealing that post-mitotic neurons can switch their identity. Whether fate reprogramming is part of normal development in the central nervous system (CNS) is unclear. While there are some reports of physiological cell fate reprogramming in invertebrates, and in the vertebrate peripheral nervous system,8 endogenous fate reprogramming in the vertebrate CNS has not been documented. Here, we demonstrate spontaneous fate re-specification in an interneuron lineage in the zebrafish retina. We show that the visual system homeobox 1 (vsx1)-expressing lineage, which has been associated exclusively with excitatory bipolar cell (BC) interneurons, also generates inhibitory amacrine cells (ACs). We identify a role for Notch signaling in conferring plasticity to nascent vsx1 BCs, allowing suit-able transcription factor programs to re-specify them to an AC fate. Overstimulating Notch signaling enhances this physiological phenotype so that both daughters of a vsx1 progenitor differentiate into ACs and partially differentiated vsx1 BCs can be converted into ACs. Furthermore, this physiological re-specification can be mimicked to allow experimental induction of an entirely distinct fate, that of retinal projection neurons, from the vsx1 lineage. Our observations reveal unanticipated plasticity of cell fate during retinal development.
Uncoupling of neurogenesis and differentiation during retinal development
03 March 2017: Peter Engerer, Sachihiro C Suzuki, Takeshi Yoshimatsu, Prisca Chapouton, Nancy Obeng, Benjamin Odermatt, Philip R Williams, Thomas Misgeld, Leanne Godinho
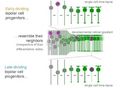
Conventionally, neuronal development is regarded to follow a stereotypic sequence of neurogenesis, migration, and differentiation. We demonstrate that this notion is not a general principle of neuronal development by documenting the timing of mitosis in relation to multiple differentiation events for bipolar cells (BCs) in the zebrafish retina using in vivo imaging. We found that BC progenitors undergo terminal neurogenic divisions while in markedly disparate stages of neuronal differentiation. Remarkably, the differentiation state of individual BC progenitors at mitosis is not arbitrary but matches the differentiation state of post-mitotic BCs in their surround. By experimentally shifting the relative timing of progenitor division and differentiation, we provide evidence that neurogenesis and differentiation can occur independently of each other. We propose that the uncoupling of neurogenesis and differentiation could provide neurogenic programs with flexibility, while allowing for synchronous neuronal development within a continuously expanding cell pool.
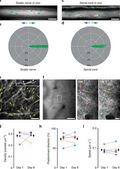
Pervasive axonal transport deficits in multiple sclerosis models
Catherine Diamante Sorbara, Naomi Elizabeth Wagner, Anne Ladwig, Ivana Nikić, Doron Merkler, Tatjana Kleele, Petar Marinković, Ronald Naumann, Leanne Godinho, Florence Martine Bareyre, Derron Bishop, Thomas MisMisgeld, Martin Kerschensteiner
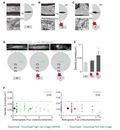
Impaired axonal transport can contribute to axon degeneration and has been described in many neurodegenerative diseases. Multiple sclerosis (MS) is a common neuroinflammatory disease, which is characterized by progressive axon degeneration-whether, when, and how axonal transport is affected in this condition is unknown. Here we used in vivo two-photon imaging to directly assay transport of organelles and the stability of microtubule tracks in individual spinal axons in mouse models of MS. We found widespread transport deficits, which preceded structural alterations of axons, cargos, or microtubules and could be reversed by acute anti-inflammatory interventions or redox scavenging. Our study shows that acute neuroinflammation induces a pervasive state of reversible axonal dysfunction, which coincides with acute disease symptoms. Moreover, perpetuated transport dysfunction, as we found in a model of progressive MS, led to reduced distal organelle supply and could thus contribute to axonal dystrophy in advanced stages of the disease.
CentrinFish permit the visualization of centrosome dynamics in a cellular context in vivo.
2014.December 01:Peter Engerer, T. Yoshimatsu, L. Godinho
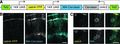
As the major microtubule organizing center in metazoan cells, centrosomes play key roles in mitotic spindle assembly, cell signaling, polarity, and motility. Additionally, centrosomal dysfunction is associated with a broad range of disorders from tumors to ciliopathies. Visualizing these organelles in vivo in a broad range of cell biological contexts would help better elucidate their roles in physiological and disease conditions. In an article on adaptive optics, Wang et al. used a novel transgenic line to image centrosomes in vivo in larval zebrafish.2 In this line, which we call CentrinFish, centrosomes and cellular membranes are labeled by spectrally distinct fluorescent proteins, permitting their simultaneous visualization. Thus, centrosome localization and dynamics can be studied within a cellular context. Moreover, because the reporters are driven by UAS cassettes, expression can be targeted to specific cell types by crossing CentrinFish to appropriate Gal4-driver lines.
An assay to image neuronal microtubule dynamics in mice
12.Sep.2014: Tatjana Kleele, Petar Marinković, Philip Williams, Sina Stern, Emily E Weigand, Peter Engerer, Ronald Naumann, J. Hartmann, Rosa Karl, Frank Bradke, D. Bishop, J.Herms, Arthur Konnerth, Martin Kerschensteiner, Leanne Godinho, Thomas Misgeld
Microtubule dynamics in neurons play critical roles in physiology, injury and disease and determine microtubule orientation, the cell biological correlate of neurite polarization. Several microtubule binding proteins, including end-binding protein 3 (EB3), specifically bind to the growing plus tip of microtubules. In the past, fluorescently tagged end-binding proteins have revealed microtubule dynamics in vitro and in non-mammalian model organisms. Here, we devise an imaging assay based on transgenic mice expressing yellow fluorescent protein-tagged EB3 to study microtubules in intact mammalian neurites. Our approach allows measurement of microtubule dynamics in vivo and ex vivo in peripheral nervous system and central nervous system neurites under physiological conditions and after exposure to microtubule-modifying drugs. We find an increase in dynamic microtubules after injury and in neurodegenerative disease states, before axons show morphological indications of degeneration or regrowth. Thus increased microtubule dynamics might serve as a general indicator of neurite remodelling in health and disease.
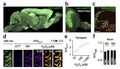
Multiparametric optical analysis of mitochondrial redox signals
20. Apri 2014:Michael Breckwoldt, F. Pfister, P.M Bradley, P. Marinković, P. Williams, Monika Brill, B. Plomer, A. Schmalz, D. C. Naumann, O. Griesbeck, M. Schwarzländer, L. Godinho, F. Bareyre, T. Dick, M. Kerschensteiner, Thomas Misgeld
Mitochondrial redox signals have a central role in neuronal physiology and disease. Here we describe a new optical approach to measure fast redox signals with single-organelle resolution in living mice that express genetically encoded redox biosensors in their neuronal mitochondria. Moreover, we demonstrate how parallel measurements with several biosensors can integrate these redox signals into a comprehensive characterization of mitochondrial function. This approach revealed that axonal mitochondria undergo spontaneous 'contractions' that are accompanied by reversible redox changes. These contractions are amplified by neuronal activity and acute or chronic neuronal insults. Multiparametric imaging reveals that contractions constitute respiratory chain–dependent episodes of depolarization coinciding with matrix alkalinization, followed by uncoupling. In contrast, permanent mitochondrial damage after spinal cord injury depends on calcium influx and mitochondrial permeability transition. Thus, our approach allows us to identify heterogeneity among physiological and pathological redox signals, correlate such signals to functional and structural organelle dynamics and dissect the underlying mechanisms.